
Two resonance structures, and I looked in several So in my opinion, it's fine to represent sulfur dioxide with these Is what our hybrid shows us and our hybrid shows us the same length between our sulfur oxygen bonds. And experimentally,īoth sulfur oxygen bonds have the same length, which So if we put in a dotted line, that bond is in between a single bond and a double bond, so like that. Over here we have a single bond, and over here we have a double bond. Line here like that, and on the right, our bond between sulfur and oxygen, let's look on the right, let me use a different color here, I'll use green. Two resonance structures would say if that bond is in between a single bond and a double bond, so we would put a dotted So if we just think about bonds, and I draw in our bonds here, the bond of sulfur to oxygen, well, if we look on the left, let me use a different color here, we have a double bond between sulfur and oxygen, on the right, we have only a single bond here. Resonance structures, so sulfur dioxide doesn't look like this, it doesn't look like this, it's a hybrid of our resonance structures. So which version of sulfur dioxide is the correct one? So in my opinion, they're both fine, because for the first version, when we draw two resonance structures, and we put in our brackets and everything, we are saying that the actual structure is a hybrid of our two Down here we have anĮxpanded valence shell, but we have zero formal charges.
So for this resonance structure, we have an octet of electrons, but we have formal charges. So we have an octet of electrons around sulfur.

If we look at this sulfur right here and we count our electrons, we have two, four, six, and then two more for a total of eight. Our resonance structures, I'll take the one on the right, we do have formal charges, which usually, our goal is If we go back up here, and we look at one of Sulfur's in the third period, so we have some d orbitals, so we can have more thanĮight electrons around sulfur so that's fine.

And that's okay to do,īecause of sulfur's position on the periodic table. We have more than eight electrons around it. If we look at sulfur, we count up the electrons around sulfur, here's two, and four, six, eight, and then 10, so we have more than eight electrons around our sulfur, so sulfur has an expanded valence shell, so this is expanded, So the advantage to this dot structure is we don't have any formal charges, so the formal charge on sulfur is zero and the formal charge on both oxygens is zero, and so this is a valid dot structure, there's nothing wrong with this dot structure, it's fine. And then we have a lone pair of electrons on our sulfur.

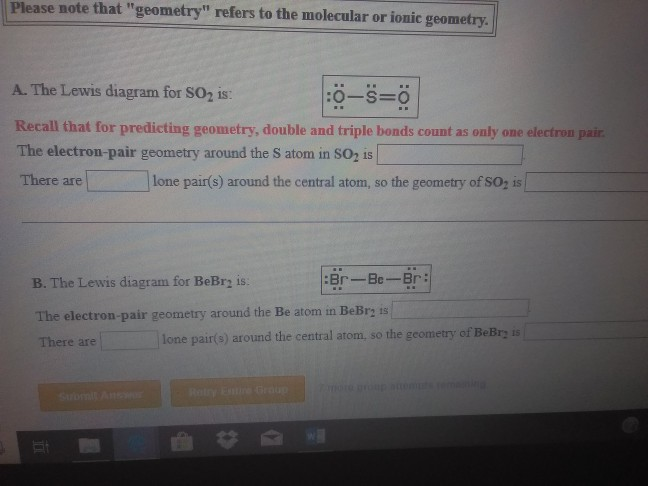
The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. So the resonance structure on the left, and the resonance structure on the right, and some people disagreed with me, and said that's not the dot structure for sulfur dioxide. In the previous video, we looked at the dot structure for sulfur dioxide, and I drew out two resonance structures.


 0 kommentar(er)
0 kommentar(er)
